The SKYTROFA Auto‑Injector
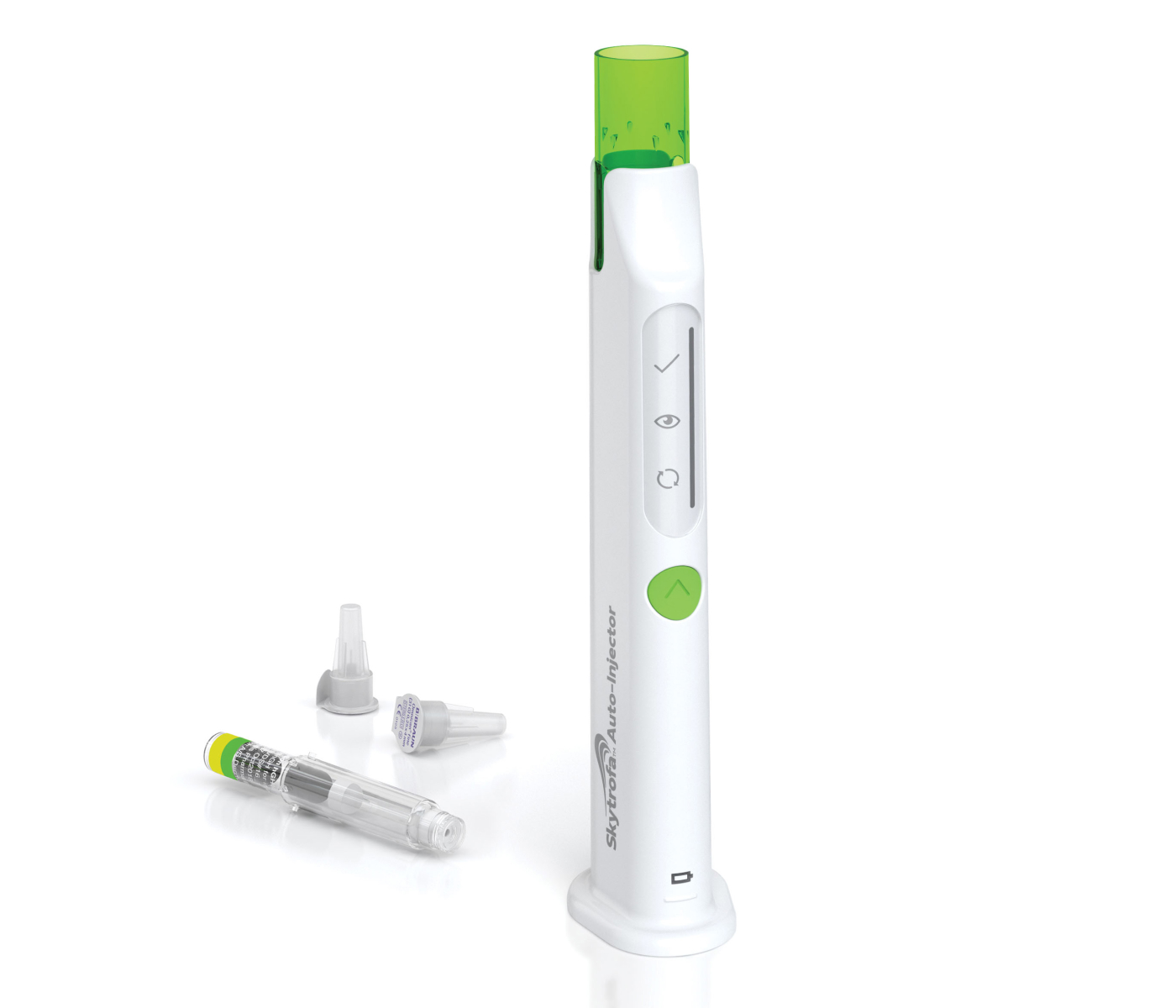
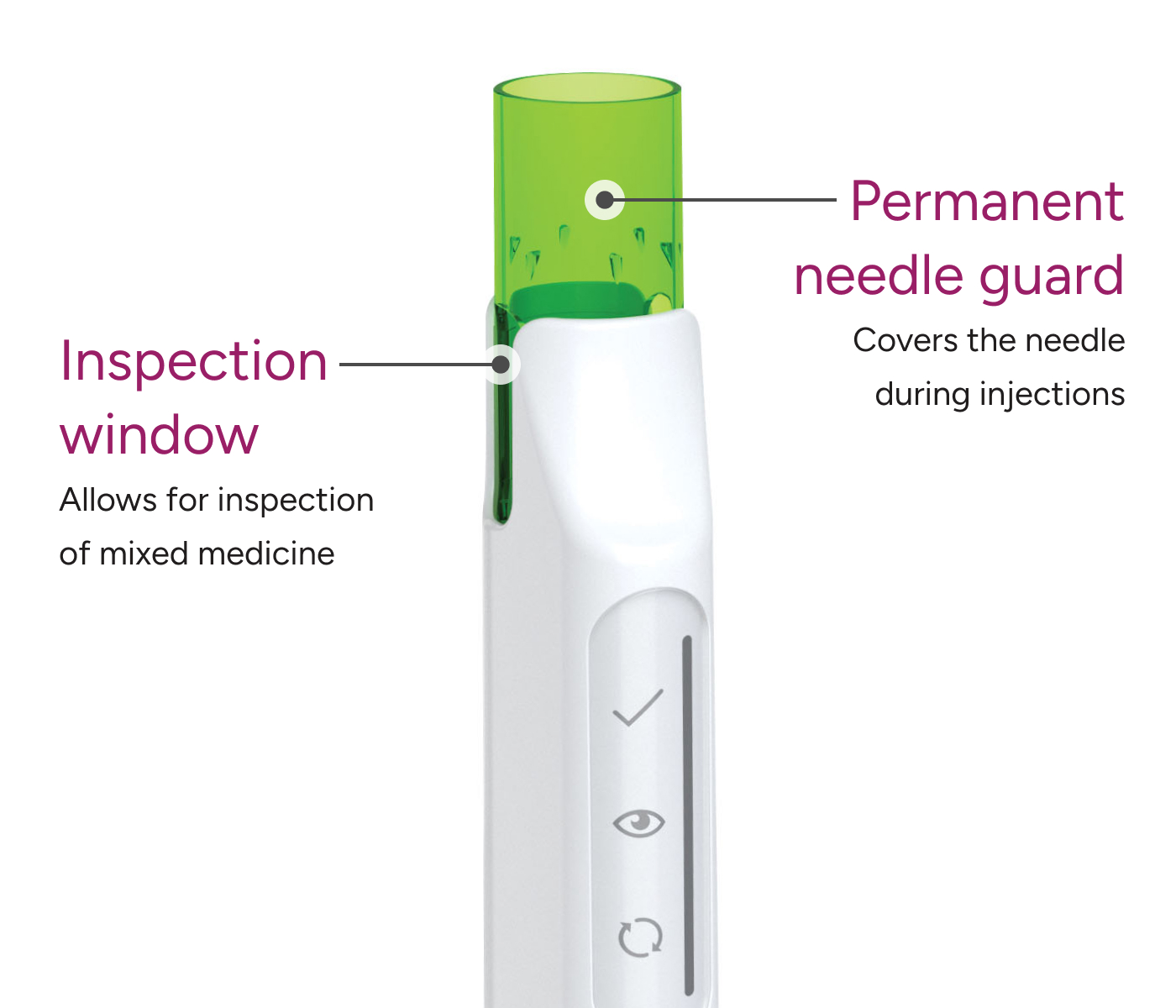
The award-winning SKYTROFA Auto-Injector was designed with patients in mind and is the only once-weekly treatment for pGHD with a permanent needle guard that keeps the needle hidden during injections.


The award-winning SKYTROFA Auto-Injector was designed with patients in mind and is the only once-weekly treatment for pGHD with a permanent needle guard that keeps the needle hidden during injections.
Its innovative design helps reduce waste with no disposable pens or batteries. A full charge powers up to 4 weeks of use (based on one injection per week) and the device is built to last up to 4 years or 210 injections, whichever comes first.


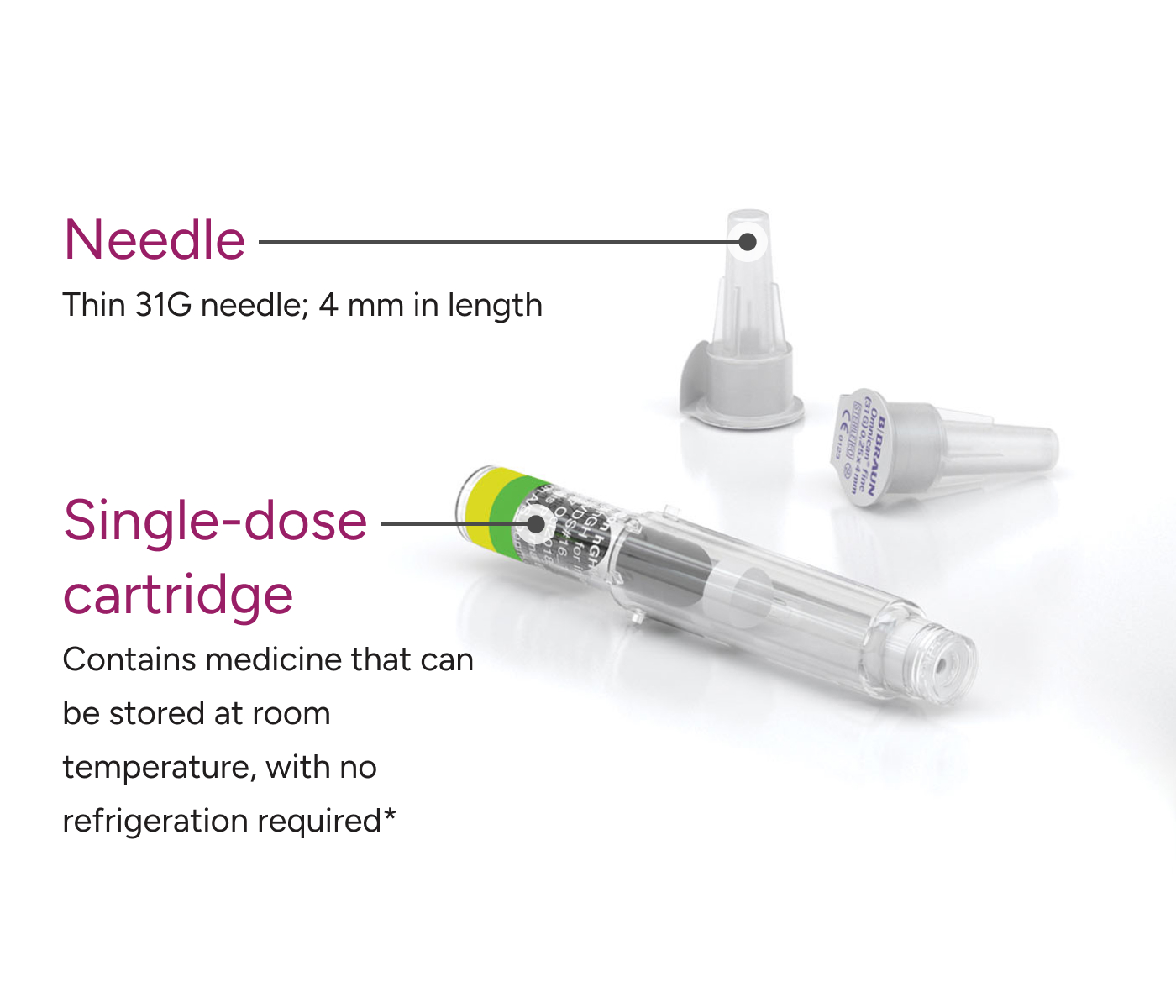
SKYTROFA cartridges do not need to be refrigerated for up to 6 months.* Each single-dose cartridge contains only the active ingredient (lonapegsomatropin-tcgd), diluent, and sterile water.
The SKYTROFA Auto-Injector delivers a single use injection with no dose dialing—delivering the full dose—while automated cues guide you through each step.
Every SKYTROFA injection uses a pre-filled cartridge with a thin, 31-gauge needle featuring a permanent guard that conceals the needle during injections.
In a clinical study* of 115 patients, patients and caregivers assessed the ease of use of the SKYTROFA Auto-Injector.

94% of patients and caregivers reported† that SKYTROFA could be administered using the auto-injector without difficulty or making a mistake.
Limitations: The auto-injector was available to US patients only. The questionnaire used in this analysis has not been validated, and the findings represent early results that have not been assessed over long-term use. Data should be interpreted with caution; no conclusions can be drawn.


–Montara, Caregiver Ambassador
There are two versions of the SKYTROFA Auto-Injector available: Generation 1 and Generation 2. Both auto-injectors work in a similar way to deliver your SKYTROFA treatment, so you can expect a familiar experience with either device. The Generation 2 auto-injector was developed to accommodate all SKYTROFA dosage strengths, including lower doses for adults with growth hormone deficiency (GHD), reflecting our ongoing commitment to supporting more people with GHD.
For detailed instructions, see the Instructions for Use.
If unsure which device your child was prescribed, contact A·S·A·P or consult this guide.
Patients who require 2 cartridges per week will need to repeat the steps shown.
It is important to be trained by an HCP on how to use your auto-injector prior to first use.
Patients who require 2 cartridges per week will need to repeat the steps shown.
It is important to be trained by an HCP on how to use your auto-injector prior to first use.

With fewer injection days than daily treatments, SKYTROFA may fit well into your families routine.
Weekly text reminders can help you and your child stay on track—even on the busiest days.
Selected for its patient-centric design in the Health Product category.
Recognized for technical innovation in advancing the field of bio/pharmaceutical manufacturing.
Awarded for outstanding, high‑quality product design.

SKYTROFA is a prescription medicine used for:
Do not take SKYTROFA if:
Tell your healthcare provider if you are pregnant or plan to become pregnant, about all of your medical conditions, and about all the medicines you take. SKYTROFA may affect how other medicines work, and other medicines may affect how SKYTROFA works.
What are the possible side effects of SKYTROFA?
SKYTROFA may cause serious side effects, including:
The most common side effects of SKYTROFA in children include: viral infection, fever, cough, nausea and vomiting, bleeding, diarrhea, stomach area pain, joint pain and arthritis
The most common side effects of SKYTROFA in adults include: swelling due to fluid build-up and low thyroid hormone
These are not all of the possible side effects of SKYTROFA. Call your doctor for medical advice about side effects. You are encouraged to report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch. You may also report side effects to Ascendis Pharma at 1-844-442-7236.
Please click here for SKYTROFA full Prescribing Information.

The site you are linking to is not controlled by Ascendis Pharma, and we are not responsible for the content presented on that site. Thank you for visiting Skytrofa.com.
Sign up to receive the latest news, resources, and information.
*Required Fields